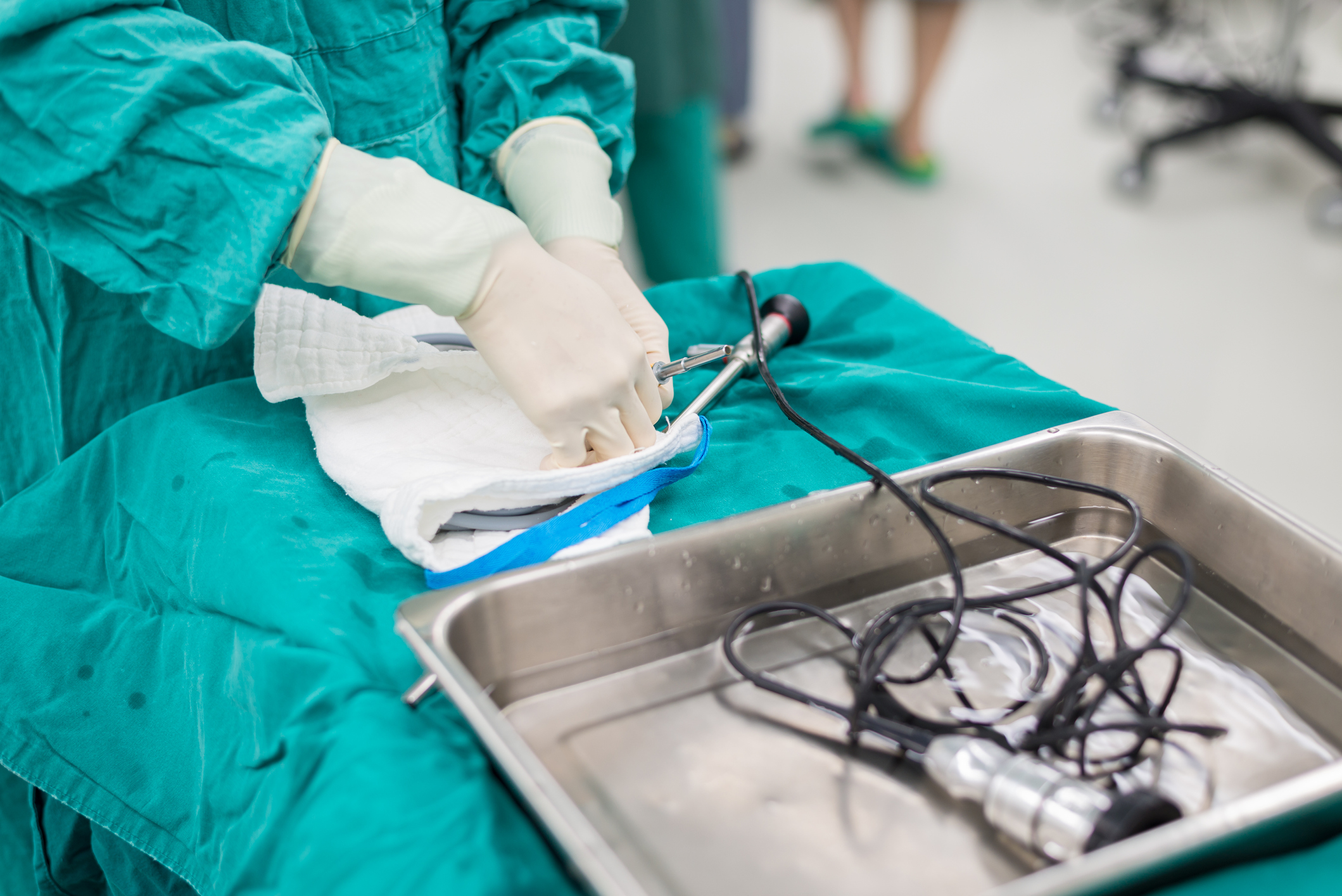
The Infection Control Consulting Services (ICCS) team has become aware of companies that develop endoscope reprocessors incorporating a "modified manual cleaning" process that "allows for quicker and easier reprocessing, automating over half of the manual cleaning steps, including flushing of the endoscope channels."
In reviewing the instructions for use (IFUs) of a company with the "automated modified manual cleaning" option, this process should not replace or provide a "shortcut" for a full manual cleaning. ICCS strongly advises organizations to continue performing a complete, manual cleaning of every scope, especially when this step is highlighted in the endoscope manufacturer’s IFUs and other reprocessing standards.
Facilities must follow IFUs developed by the scope manufacturers, scope reprocessor equipment manufacturers and manufacturers of cleaning and high-level disinfection (HLD) products. In addition, standards and recommendations of nationally recognized organizations, associations and agencies, if selected by the facilities, must be taken into consideration.
For example, the Centers for Disease Control and Prevention (CDC) through the Healthcare Infection Control Practices Advisory Committee (HICPAC) released recommendations in 2016 with an update in 2017 titled "Essential Elements of a Reprocessing Program for Flexible Endoscopes." Its recommendations include the following: "Manual cleaning is the most critical step in the disinfection process since residual organic material can reduce the effectiveness of HLD and sterilization."
AORN, SGNA and AAMI provide standards for reprocessing scopes, which also include manual cleaning in their guidance.
ICCS advises facilities to perform scope cleaning verification as another critical step in assuring reprocessing effectiveness. There are several verification methods and products available to assist facilities with the goal of effective reprocessing of scopes.